Corina Intrauterine Drug-Eluting System approved in China as a novel treatment for patients with moderate-to-severe intrauterine adhesions (IUA)
 2024-08-27
2024-08-27
 Puyi (Shanghai) Biotechnology Co., Ltd
HaiPress
Puyi (Shanghai) Biotechnology Co., Ltd
HaiPress
The World's First-in-Class Intrauterine Drug-Eluting System
SHANGHAI,Aug. 23,2024 -- Corina Intrauterine Drug-Eluting System("Corina"), developed by Yipurun (Shanghai) Biotechnology Co.,Ltd. ("YPR"),has received approval in China (G.X.Z.Z. 20243181461) for use in patients with moderate-to-severe intrauterine adhesions (IUA)who undergo transcervical resection of adhesions (TCRA). The approval for this indication is based on a randomized controlled clinical trial that demonstrated Corina's superiorefficacy compared to the standard-of-care. Corina is a first-in-class treatment focused on promoting female reproductive health through the prevention of intrauterine re-adhesion risk post TCRA,and the improvement of endometrial growth.
Intrauterine adhesions (IUA) are the formation of scar tissues in the endometrial cavity as a result of damages to the endometrial basal layer of the uterus,which leads to the partial or complete occlusion of the cervical canal and uterine cavity. Clinically,IUA can cause a range of symptoms such as reduced menstrual flow,amenorrhea,infertility and recurrent miscarriage– therebyposing a serious threat to the reproductive health of women of childbearing age. Currently,the incidence rate of IUA in China remains high,and continues to grow due to an increased number of intrauterine interventions. Research in this field show the incidence rate of IUA in China to be as high as 25% in women who have had prior abortionor curettage procedures [1]— this has become a key driver of reduced menstrual flow and secondary infertilityin women of childbearing age.As a standard surgical procedure to treatIUA,TCRA separates the adherent tissues and restores the anatomical shape of the uterine cavity. Surveys show1.6 to 1.8 million patients in China undergoTCRA every year,but the recurrence rate of IUA after surgery is still as high as 62.5%[2]; and the pregnancy success rate of patients after TCRA surgery is only 22.5%~33.3%[3-4].Over the years,a variety of adjuvant therapies,such as uterine cavity isolation barriers and hormonal treatments,have emerged with limited efficacy.
Corina is an innovative drug-device-combination therapy made from non-degradable,medical grade material,carrying the active ingredient estradiol. Designed to conform to the shape of the female uterus,Corina acts as a physical barrier upon insertion,and enables targeted delivery of estradiol to the endometrium. With YPR's proprietary controlled-release technology,Corina can continuously releasethe active substance up to 60 days post procedureto promote endometrium growth.
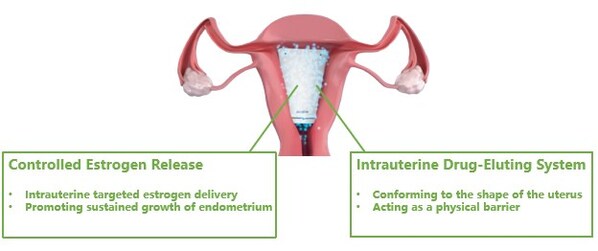
Corina acts as a physical barrier upon insertion,and enables targeted delivery of estradiol to the endometrium
The registration study results of Corinahave demonstrated statistically significant and clinically meaningful reduction in intrauterine adhesion at 60 days post procedure,compared to standard-of-care therapy. Corina has also shown meaningful improvement in endometrial thickness from baseline at 60 days post procedure,compared to standard-of-care therapy.
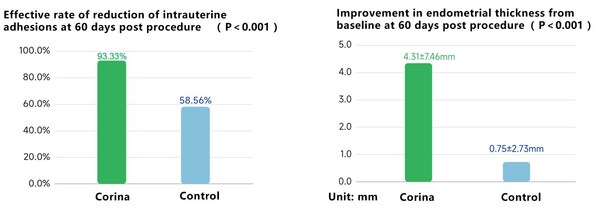
Evaluating effectiveness data from clinical trials
About Puyi Biotech
Located in the Zhangjiang Hi-Tech Park in Shanghai,Yipurun (Shanghai) Biotechnology Co.,Ltd. ('YPR') is a wholly-owned subsidiary of Puyi (Shanghai) Biotechnology Co.,Ltd. ("Puyi Biotech"or "Group"). Leveraging its knowhow in biomedical material scienceand its proprietary drug-eluting technology,Puyi Biotech and YPR have developed an innovative R&D pipeline of high-end implantable therapeutics. Since its founding in 2012,Puyi Biotech has focused on providing innovative solutions to address unmet clinical needsacrosswomen's health,ENT (ear,nose and throat),and medical aestheticscategories. The Group has a number of renowned institutions as investors including Goldman Sachs Alternatives,Legend Capital and Northern Light VC.
References
[1] 2015 Chinese expert consensus on the clinical diagnosis and treatment of intrauterine adhesions
[2] YuD,WongYM,CheongY,et al.Asherman syndrome: one century later[J].Fertil Steril,2008,89(4):759–779.
[3] YuD,LiTC,XiaE,et al.Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman's syndrome[J].Fertil Steril,89(3):715–722.
[4] RoyKK,BaruahJ,SharmaJB,et al.Reproductive outcome following hysteroscopic adhesiolysis in patients with infertility due to Asherman's syndrome[J].Arch Gynecol Obstet,2010,281(2):355–361.
Contact Us
Address: Room 2206,No. 396,Lishizhen Road,Pudong New Area,Shanghai,China
Company Contact Tel: +86-21-50805520,Merchants Phone: +86-21-58556388
Website: en.puyibio.com
Email: service@puyibio.com
Newest
-

iCAUR Makes Strategic Entry into the Middle East: Announcing V27's Global Launch in UAE
-

iCAUR Makes Strategic Entry into the Middle East: Announcing V27's Global Launch in UAE
-

MEXC Ranks No. 1 in XAUT Perpetual Volume Globally, Demonstrating Strong Liquidity and User Activity
-

ZHAO Shanrui's Sales Symbiosis Practice
